Unfortunately, all of the published vaccination studies to date have not included pregnant women.
- In general, pregnant women are more susceptible to viral respiratory infections due to inherent immunologic changes associated with pregnancy.
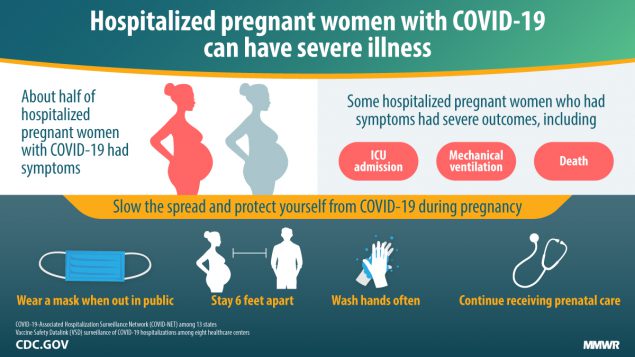
- Although the majority of pregnant women with COVID-19 develop mild to moderate symptoms, similar to that of age-matched, non-pregnant patients, emerging data suggest that pregnant women may carry a higher risk of severe disease from COVID-19, including a greater risk of hospital admission, need for supplemental oxygen, and intensive care unit admission.
These risks are higher in those with specific comorbidities including cancer, chronic kidney disease, heart conditions (heart failure, coronary artery disease, cardiomyopathies), immunocompromise, obesity (body mass index ≥ 30 kg/m2), sickle cell disease, smoking, and type 2 diabetes mellitus.
- In general, pregnant women are more susceptible to viral respiratory infections due to inherent immunologic changes associated with pregnancy.
- Although the majority of pregnant women with COVID-19 develop mild to moderate symptoms, similar to that of age-matched, non-pregnant patients, emerging data suggest that pregnant women may carry a higher risk of severe disease from COVID-19, including a greater risk of hospital admission, need for supplemental oxygen, and intensive care unit admission.
These risks are higher in those with specific comorbidities including cancer, chronic kidney disease, heart conditions (heart failure, coronary artery disease, cardiomyopathies), immunocompromise, obesity (body mass index ≥ 30 kg/m2), sickle cell disease, smoking, and type 2 diabetes mellitus.
There are currently 3 approved COVID19 vaccines available in the US:
- Two of the vaccines (Pfizer/BioNTech and Moderna) are 'mRNA' vaccines. We discussed that this means that a small amount of genetic material (RNA) that our own cells use as a 'code' to make the spike protein that is the same as the spike protein on the outside of a SARS-CoV-2 viral cell. Our body makes this spike protein and then trains itself to create antibodies against the spike protein. If, in the future, we are exposed to the SARS-CoV-2 virus, our body's antibody's will attack the COVID-19 virus before it can multiply enough to make us sick. This means that the vaccine does NOT carry any part of the actual SARS-CoV-2 virus and is not an 'inactivated' or 'weakened' version of the virus. No genetic changes are made in one's cells after receiving the vaccine. All components of the vaccine itself are degraded naturally by the body.
- The most recently approved vaccine is manufactured by Johnson & Johnson (J&J), otherwise known as the "Janssen vaccine" and is an adenovirus vaccine. This vaccine uses a weakened live pathogen (adenovirus, cause of the common cold) as a way to deliver a small amount of genetic material (DNA) from the COVID-19 vaccine. This genetic material for COVID-19 does not replicate in the body. Similar to the RNA vaccine, it enters our own cells but uses the adenovirus vector to do so, and then our own cells use the DNA as a code to make the spike protein that is the same as the spike protein on the outside of a SARS-CoV-2 viral cell. It then functions very similar to mRNA vaccines as described above.
- The studies of the two mRNA vaccines demonstrated approximately 95% efficacy, and for the Johnson&Johnson vaccine, the efficacy within the group of patients randomized in the US was 72%. The chance of 'severe' COVID-19 infection was lower in those who got the vaccine, and though the efficacy of the J&J vaccine was slightly lower in trials, there were no deaths related to COVID-19 and no cases of severe COVID-19, and the J&J vaccine was tested 'later' than the 2 mRNA vaccines at a time when the COVID-19 variants were more common. This has led some experts to conclude that efficacy of all 3 vaccines may indeed be similar.
We advise that pregnant women speak with their healthcare providers re: the COVID vaccine. In addition, the following infographic may help provide additional guidance regarding whether or not to receive the vaccine.
(credit: Baystate health/UMass Medical School)
(credit: Baystate health/UMass Medical School)
Your browser does not support viewing this document. Click here to download the document.